TXL Human Clinical Trials- Breast Cancer
The Drug Dictionary C-88275 was officially issued by NCI...
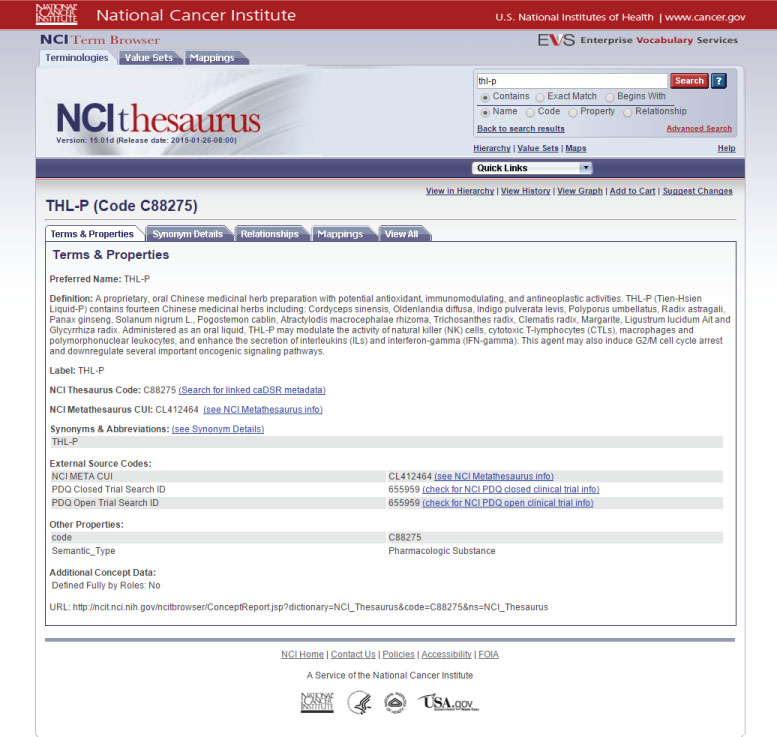
Definition of THL-P by the National Cancer Institute
A proprietary, oral Chinese medicinal herbal preparation with potential antioxidant, immunomodulating, and antineoplastic activities. THL-P (Tien-Hsien Liquid-P) contains fourteen Chinese medicinal herbs including: Cordyceps sinensis, Oldenlandia diffusa, Indigo pulverata levis, Polyporus umbellatus, Radix astragali, Panax ginseng, Solanum nigrum L., Pogostemon cablin, Atractylodis macrocephalae rhizoma, Trichosanthes radix, Clematis radix, Margarite, Ligustrum lucidum Ait and Glycyrrhiza radix.
Administered as an oral liquid, THL-P may modulate the activity of natural killer (NK) cells, cytotoxic T-lymphocytes (CTLs), macrophages and polymorphonuclear leukocytes; it enhances the secretion of interleukins (ILs) and interferon-gamma (IFN-gamma). This agent may also induce G2/M cell cycle arrest and down regulate several important oncogenic signaling pathways.
Human Clinical Trial on Breast Cancer by National Taiwan University Hospital
The official website of US National Institute of Health has officially announced and proved the data collected from the human clinical trial of THL-P.
Evidence-Based Complementary and Alternative Medicine Volume 2012. Article ID 803239, 8 pages United Kingdom.
Memorial Sloan Kettering
Why Is Chinese Complex Herbal Composition Tian Xian Liquid Included In The Medical Thesaurus of International Importance On Cancer?
Tian Xian Liquid has continued to develop and show better results for more than twenty years- thanks to a Professor from Stanford University in the United States. Between 1996 or 1997, one of the major board members of Tian Xian Liquid organization visited this Professor in the United States on his friend’s recommendation, hoping that this Professor would offer support and advice to Tian Xian Liquid showing his scientific concern. However, after he had handed his business card to the Professor and was going to introduce himself, the Professor, unexpectedly, tossed the business card away right after seeing the words ‘cancer killer’ printed on the card. After condemning Tian Xian Liquid as a rustic folk medicine, he showed his guest the door. This incident provoked the determination of the team, to present the beneficial qualities of Tian Xian Liquid on the world stage of medicine. This also helped a series of experiments to be carried out since 1999.
So far, the medicinal qualities of Tian Xian Liquid have been examined in more than 20 research cases, as well as in 13 official articles in international medical journals, and two human subject clinical trials have been conducted. Tian Xian Liquid has also been included in the Medical Thesaurus of one of the world’s largest cancer research centers, namely the National Cancer Institute (NCI) of the United States under the code C88275. NCI also published information about the efficacy and characteristics of Tian Xian Liquid on their website, pointing out that Tian Xian Liquid possesses the ability to modulate the activity of natural killer (NK) cells, T-Lymphocytes cells (CTLs), macrophages and polymorphonuclear leukocyte, and enhance the secretion of interleukin (ILS) and IFN-gamma interferon; There it is also stated that Tian Xian Liquid may also induce G2/m cell cycle rest, and downregulate several important oncogenic signaling pathways.
Do you know that Tian Xian Liquid is the first Chinese complex herbal composition that is included in NCI’s Medical Thesaurus since its establishment? Such an achievement is unprecedented in the history of traditional Chinese medicine.
Why Has Such Good Chinese Complex Herbal Composition Not Been Accepted By Hospitals?
The stereotypical image most people have of Chinese medicine as a folk remedy or secret prescription might already be familiar to you. In fact, many people do have the impression that the major function of Chinese medicine is only to improve health in general. Nowadays, keeping in mind the fact that Western medicine has achieved a dominant position in world medical practice, we could still expect after the resurgence of traditional Chinese medicine that it would gradually attract more attention. However, we should be aware that Chinese medicine has a long way to go before being formally accepted into the contemporary medical system.
In fact, prior to the acceptance of any new medicine by hospitals, it had to undergo different basic experiments, test on animals, and then gradually proceed to larger and more complex phases of clinical trials on humans. It took many years to complete all the four stages of clinical trials. Currently, Tian Xian Liquid has already completed the second phase of the clinical trials, and has proven to be safe and effective. The result of the experiments indicates that Tian Xian Liquid has the potential of becoming a therapeutic medicine that targets the cancer stem-like cells, and can play the role of a complementary therapy, thus reducing the risk of cancer recurrence.
What is Human Clinical Trial?
Clinical trials refer to a systematic study of the influence of a medication on the human body, in order to examine and verify the function and side-effects of a particular medicine, as well as assess its safety and therapeutic efficacy. It is usually divided into four phases, and each phase has certain requirements concerning its basic principles and techniques.
The National Taiwan University Hospital Carries Out Human Subject Clinical Trials For Breast Cancer
In 2009, the National Taiwan University Hospital included Tian Xian Liquid into its research program, in order to measure the safety and effectiveness of the potent Tian Xian Liquid- TXL-P in patients with metastatic breast cancer. This research program used the quality of life, the changes in immune system, as well as the changes in the size of the tumour of the subject experimented on as the index to evaluate the clinical efficacy of TXL-P. All forty-four patients that participated in the program had to meet the strict selection criteria, including the following:
- The patient had to be in the final stage (stage 4) of breast cancer.
- The patient had to have undergone surgery, chemo or radiation therapy and yet shown no signs of improvement.
- The patient had to have multiple metastases of cancer cells, including the brain, lungs, liver, and lymph nodes. Every patient that participated in the program had two or more signs of metastasis.
- The patient had to have life expectancy of four weeks or more.
The trial was conducted in a randomized double-blind, placebo-controlled approach for a treatment period of 24 weeks. The placebo and controlled groups consisted of 14 and 30 patients respectively. The trial results showed that the patients’ quality of life, physical function, and cognitive abilities had significantly improved; while the patients’ emotions, fatigue, as well as common side effects were relieved. The pains, loss of appetite, insomnia and constipation problems were also softened. Besides, the test results indicated that Tian Xian Liquid has immunomodulatory function, as well as lymphocytes activation function.
♥ Follow our facebook page for more information: https://www.facebook.com/tianxian/



